This is a jar full of only water (liquid and vapor). It boils at any temperature when you apply something cold enough to the top, like ice.
cross-posted from: https://lemmy.sdf.org/post/2697716
I put water in a jar and sealed it while it was boiling, and now it boils at any temperature. Super fun demo to try.
I’ve read the video description several times, and I still don’t understand. This bit in particular:
Whenever there is a heat transfer from the bulk water to the lid (condensing the vapor), the water boils, no matter the absolute temperature.
Is the idea that water condenses at a lower energy state, so when cold is applied, the water vapor turns to liquid? If so, what does that have to do with the water boiling?
I don’t understand.
When the water vapor inside the jar comes in thermal contact with the ice outside, it condenses and precipitates. This decreases the vapor pressure inside the jar, which then causes the water to boil.
Boiling is not just a temperature-based phenomenon, it’s also a pressure-based one: a water body maintains an equilibrium between liquid water and water vapor right above its surface. If you remove the water vapor from above the surface, it decreases the vapor pressure and shifts the equilibrium away from the liquid state, which is essentially boiling. Note that this is different from evaporation since the liquid water is not using heat from an external source to vaporize. You can also see this in daily life, for example, in that water boils at a different temperature on mountains due to pressure difference.
So, the way this trick works is due to bottling at high(ish) temperature and letting it cool to form a vacuum, and then cooling it further creates a negative pressure ?
Exactly. it was bottled at atmospheric pressure while it was boiling, so 1 atm and 100 degrees C. Check this graph to see the relationship between the water’s temperature and it’s pressure in the jar (since there is no air, only water vapor). If the vapor is condensed, then the pressure drops below the curve on the graph, that is, the pressure in the jar is lowered below the vapor pressure of the water. Any time the pressure is below the vapor pressure, the water will boil, releasing vapor, until the pressure is equal to the vapor pressure. The pressure does not become negative, it is still positive, just lower than the vapor pressure at the given temperature. You can get below the vapor pressure curve by changing the temperature too, which is what we usually do when boiling water at a pressure near 1 atm (760mmHg)
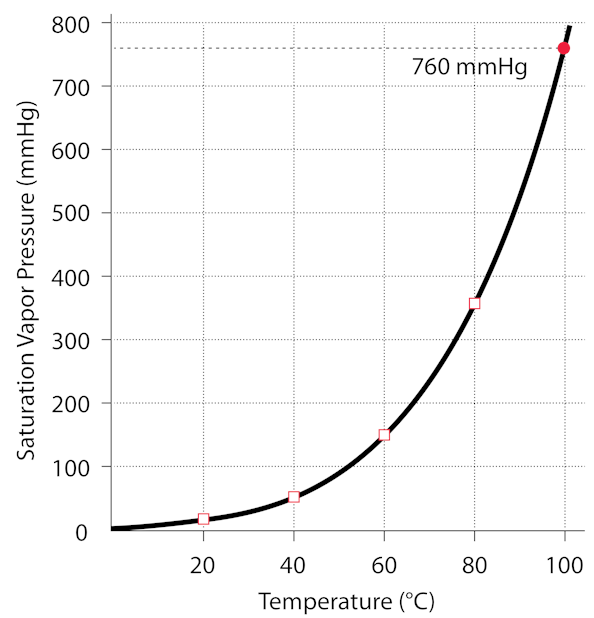
http://hyperphysics.phy-astr.gsu.edu/hbase/Kinetic/watvap.html#c2
(1 atmosphere is ~760mmHg)
a slight aside, there is an important difference between the total pressure of the air, and the partial pressure of water vapor in the air. Inside the jar, the two are equal, but in a dry location (not humid) the partial pressure of water vapor is usually less than the vapor pressure of water at that temperature, but since the total large pressure of the atmosphere would not allow a pocket/bubble of very low pressure water vapor to form inside the bulk water, the water cannot boil, but it will evaporate at the surface anyway until the partial pressure of water is equal to the vapor pressure (very humid).
You are sollidly correct, and your arguments are correct, but TMI (Too Much Information) applies (to me). Be well, indeed live long and prosper…
Paint it as a chemical reaction in order to understand its equilibrium state. We basically have:
H2O (gas) ⇌ H2O (liquid)
By sealing the jar with the water already boiling, we initialize the system to be in a state with equal(ish) amount of both liquid and gas. Then we allow the system to cool down so that the liquid water is no longer boiling. Now the system sits at an equilibrium between liquid and gas states.
Now, when we put ice on top of the jar, the water vapor condenses and gets converted to liquid, pushing the equilibrium to the right. But this decreases the overall pressure in the system since fewer particles now occupy the volume above the liquid’s surface. This is essentially the system trying to pull itself back towards the original equilibrium i.e. towards the left of the equation, which it does by making more water vapor i.e. boiling.
This reaction-like picture helps in visualizing the system better in some cases, so I tried to add it alongside the pressure dynamics scenario. You may be interested in Le Chatelier’s principle if you prefer this.
Anything for posterity
That is a cool chemistry experiment! Thank you for sharing!
Now I wonder if the pressure inside is low enough that you could reach the water’s triple point If you cool it down to 0,01°C
Yes definitely. The pressure will drop along the vapor pressure curve all the way to the triple point, gently boiling all the way if you remove the heat from the vapor and not directly from the water.
Thank you for the answer. I’m tempted to try it, but I’m guessing this is not something you can easily do at home.
Do it! it is easy to do at home! Just wear some gloves and safety glasses, those jars can easily shatter during the heating process if you use too hot of a heat source. I recommend a glass top electric stove, or put some kind of metal plate between your jar and the burner to help spread out the heat. Once you seal the jar, take it off the heat right away, so it doesn’t build pressure. I boiled mine for a few minutes before sealing to try and get some of the devolved gasses out, and lightly set the lid on top to help the steam push out all the air.
Hmm. I wonder if you could make a higher-quality vacuum this way by adding a getter. You’d think it would still be safe-ish with reactive metals because bulk liquid water never needs to be anywhere near the chamber (air will remove heat too, just somewhat slower).






